Watching a Virtual Sneeze Can Change Your Immune System
What the brain-immune connection reveals about Alzheimer’s, depression, and beyond
What if I told you simply watching someone act sick, without ever touching them or breathing the same air, could change your immune system?
In 2025, Trabanelli and colleagues put that idea to the test. They gave healthy volunteers virtual reality headsets and dropped them into a simulated room. In front of them, an avatar shuffled in, sneezing, coughing, shivering—all the classic signs of being sick.
The participants never had any physical contact with the avatar. When the researchers drew blood before and after the VR exposure, they found something surprising. The number of innate lymphoid cells—frontline immune cells—had gone up, the same pattern you see after a flu shot. Brain scans (fMRI and EEG) told the other half of the story: the “infectious” avatars had activated the hypothalamic–pituitary axis, the brain’s stress–immune switch. Neutral or fear-inducing avatars didn’t have the same effect.
From the perspective of classic germ theory, which says the immune system only springs into action in response to actual germs, or pathogens, this shouldn’t happen. But it does. And findings like this are what neuroimmunology is about: how the brain and the immune system talk to each other.
Their conversation is constant, surprisingly detailed, and it shapes everything from mood to memory to how we age. Understanding neuroimmunology has already changed how we think about Alzheimer’s, depression, and autoimmune disorders. It may also pave the way for therapies for diseases that resist current treatments.
The brain is sealed off from the rest of the body (or so we thought)
For most of the 20th century, textbooks taught that the brain was strictly “immune privileged.” People thought the brain was protected from the chaos of the immune system. No patrols of antibodies or immune cells wandering in from the bloodstream, no inflammation unless something major had gone wrong, such as a severe brain injury or a major infection.
This belief rested on two pieces of evidence.
In 1948, Peter Medawar noticed that tissue grafts placed in the brain survived much longer than grafts in skin or muscle, which the immune system destroyed almost immediately. To scientists at the time, this was evidence that the brain was somehow shielded from the immune system’s attacks.
The blood–brain barrier (BBB) was first hinted at in 1885, when the German scientist Paul Ehrlich injected dye into an animal’s bloodstream and saw it stain every organ except the brain. His student, Edwin Goldmann, later injected dye directly into the cerebrospinal fluid of animal brains and found it stayed there, unable to leak out into the rest of the body. These results suggested some kind of compartmentalization between the brain and the body, perhaps upheld by a tight barrier around the brain.
Seeing the blood-brain barrier up close
For decades, the BBB was more concept than confirmed structure. This was the case until Reese and Karnovsky used electron microscopy to visualize brain capillaries at high resolution in 1967.
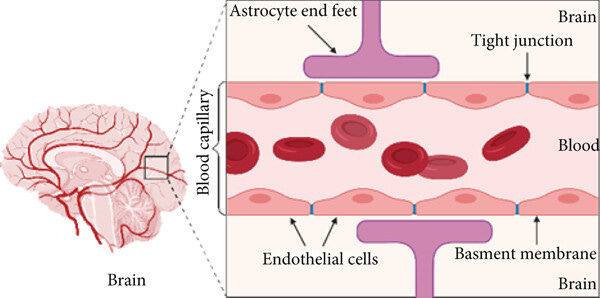
The endothelial cells lining the blood vessels were zipped shut by tight junctions (specialized cell-to-cell seals), which created a continuous barrier that allowed very few things to cross. And unlike the leakier capillaries in other parts of the body, brain vessels had far fewer transport vesicles—tiny sacs that move materials in and out—cutting down even more on unwanted exchange.
Cracks in the wall
Still, there were hints that the brain wasn’t as cut off as scientists once believed. Microglia, cells known to live in brain tissue, behave like immune cells in our blood, and even immune cells from the rest of the body could slip into the healthy central nervous system (CNS) using the same routes they take into other organs, though they do so at a much lower rate into the CNS.
For years, these sightings were seen as rare breaches, exceptions to a strict rule of separation. That picture changed in 2014, when Antoine Louveau, working in Jonathan Kipnis’s lab, then at the University of Virginia, used high-resolution microscopy and fluorescent tracers in live mice to map how fluid actually drains from the brain.
To their surprise, they saw thin, vessel-like channels running alongside the dural sinuses—large veins embedded in the brain’s protective membranes. These hidden pathways shuttled immune cells and fluid straight to nearby lymph nodes, linking the brain to the body’s immune network.
These meningeal lymphatic vessels had been overlooked for years, partly because they hug the edges of major blood vessels and are nearly invisible without modern imaging tools. As you can see in the GIF below, these lymphatic vessels can be visualized with MRI.
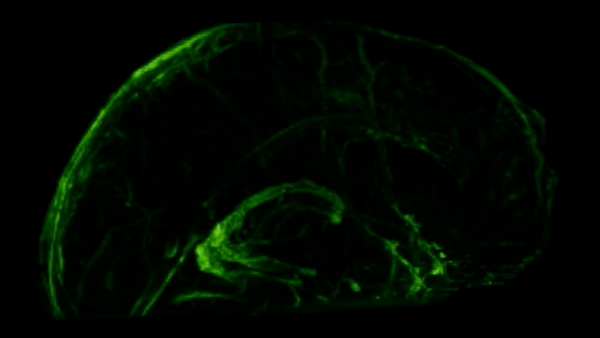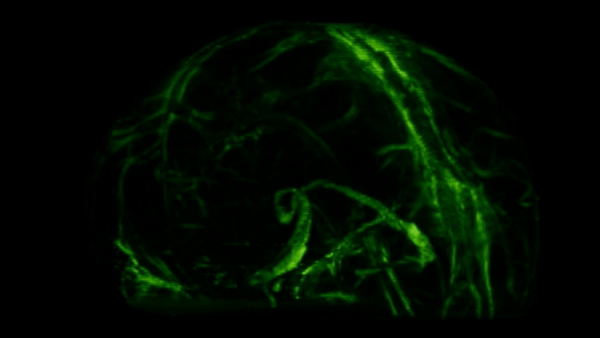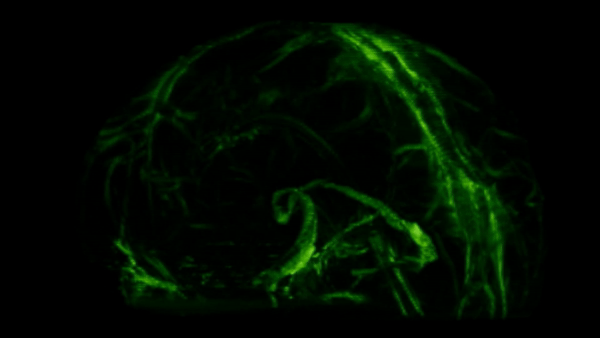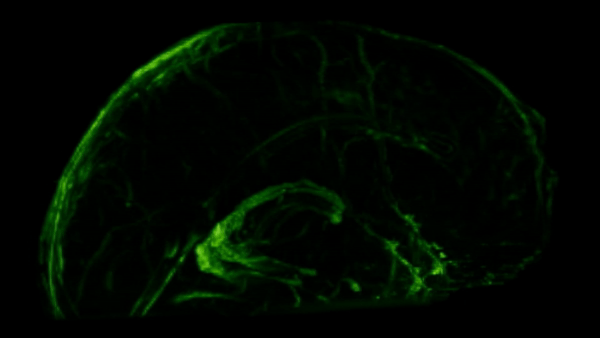
So we’ve established there are immune cells in the CNS, but what are they actually doing there?
The answer is not simply “fighting infections.” Immune cells play many roles in the CNS and often have little to do with pathogens.
What immune cells actually do in the brain
Microglia: the brain’s resident immune cells
Microglia arrive in the brain before birth and stay for life. They’re macrophage-like cells in that they respond to injury and infection by engulfing debris, but much of their work is in routine maintenance.
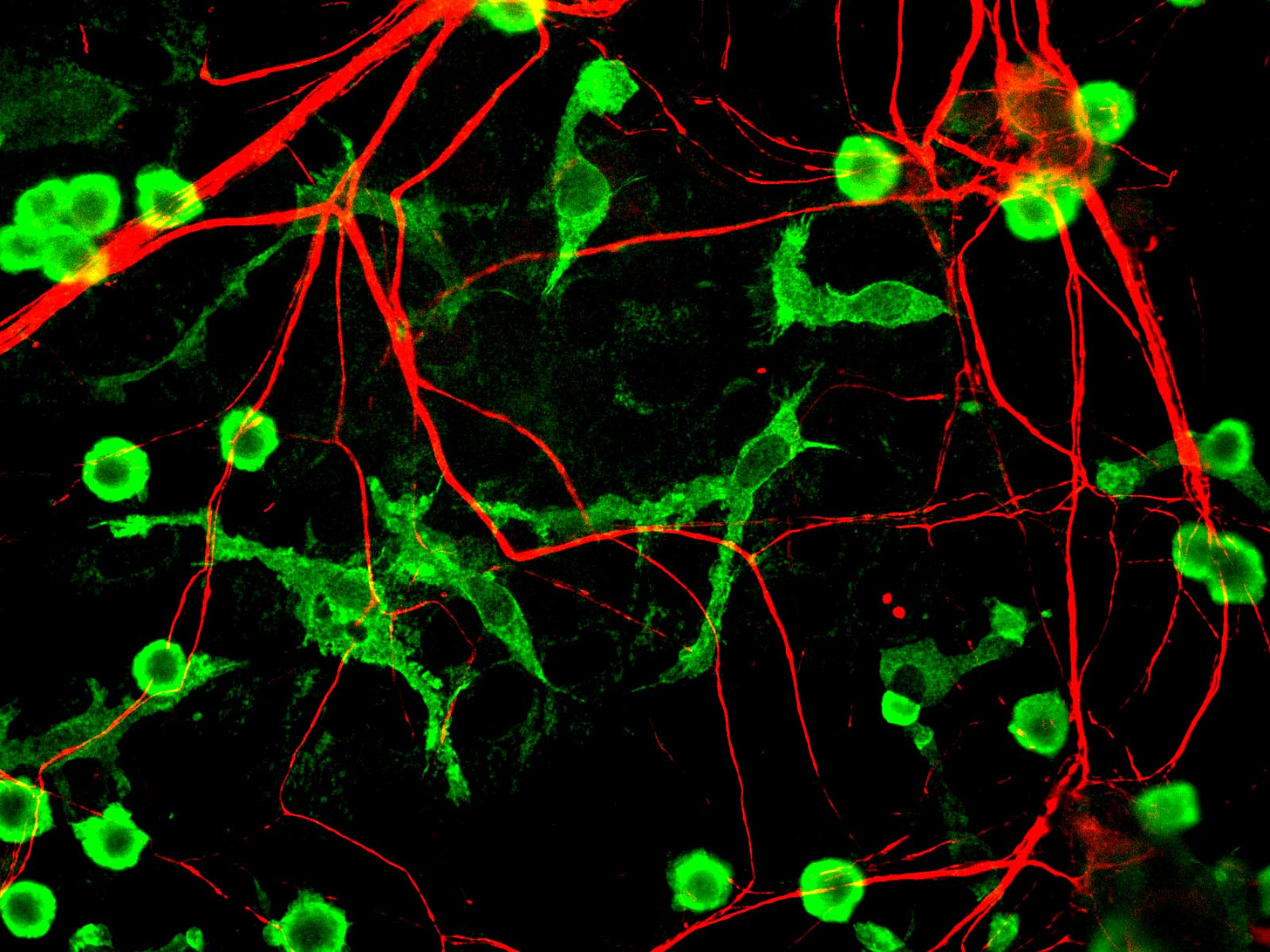
Specifically, they shape the brain’s wiring by trimming extra synapses, keep its signals in balance, and switch into repair mode to help nerves grow back after injury.
Meninges as an immune outpost
The meninges—three layers of membrane around the brain and spinal cord—aren’t just passive protection. These layers contain T cells, which coordinate immune responses; B cells, which make antibodies; dendritic cells, which present antigens; and other immune cells that help guard against infection.
If you’ve heard of meningitis, that’s the inflammation of these very membranes.
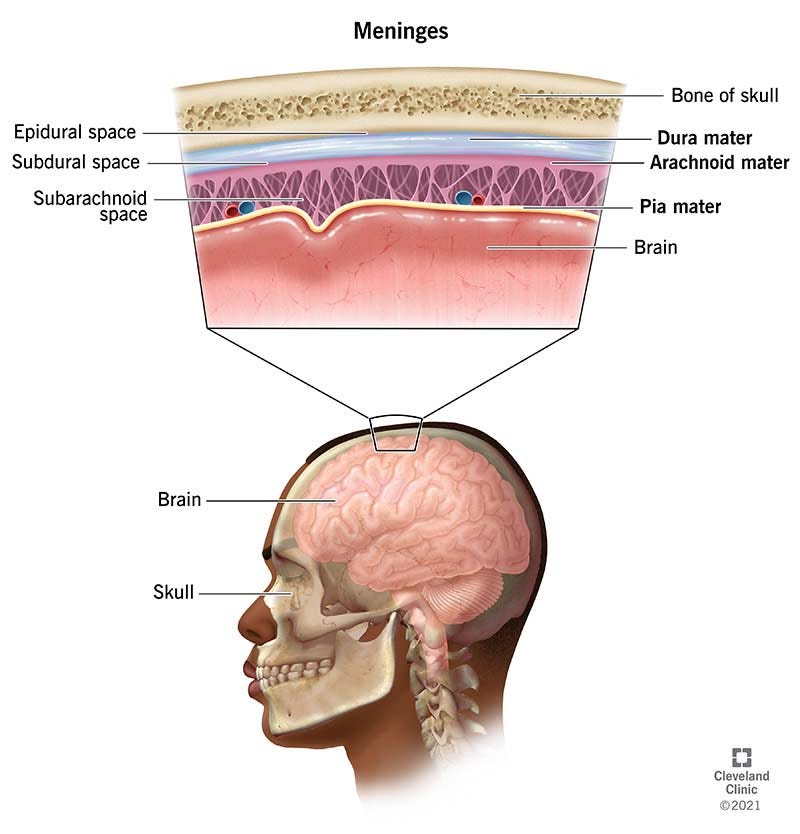
Sitting just outside the blood–brain barrier, meningeal immune cells keep watch over both the cerebrospinal fluid and messages coming from the blood. From this vantage point, they can quickly spot signs of infection or injury and respond, either by taking action locally or by calling in help from the body’s other immune organs.
Peripheral immune cells crossing over into the central nervous system
Most of the time, the blood-brain barrier limits the entry of immune cells, but the barrier’s permeability is not fixed. Infection, injury, and certain stressors can allow peripheral cells to enter.
One striking example: In chronic social stress experiments1, a type of white blood cell called CCR2+ monocytes are recruited from the blood into a brain region involved in motivation and reward, the nucleus accumbens. These monocytes release an enzyme that remodels the extracellular matrix and alters synaptic structure. The result: changes in behavior linked to depression and anxiety.
Peripheral immune cells communicate with peripheral nerves
Peripheral immune cells can also influence neurons without entering the brain. They do this by releasing signaling molecules (cytokines, chemokines, and in some cases, neurotransmitters). An example is acetylcholine-producing T cells in the spleen, which participate in the inflammatory reflex—a vagus-nerve-mediated feedback loop that tones down excessive inflammation.
The spleen is not the only organ where immune and neural signals intersect. In the skin, sensory neurons detect injury or infection and trigger local immune cells to release antimicrobial peptides, boosting the barrier’s defenses. The figure below shows how nerve endings in the skin send signals that recruit macrophages, T cells, and other defenders to the site of injury.
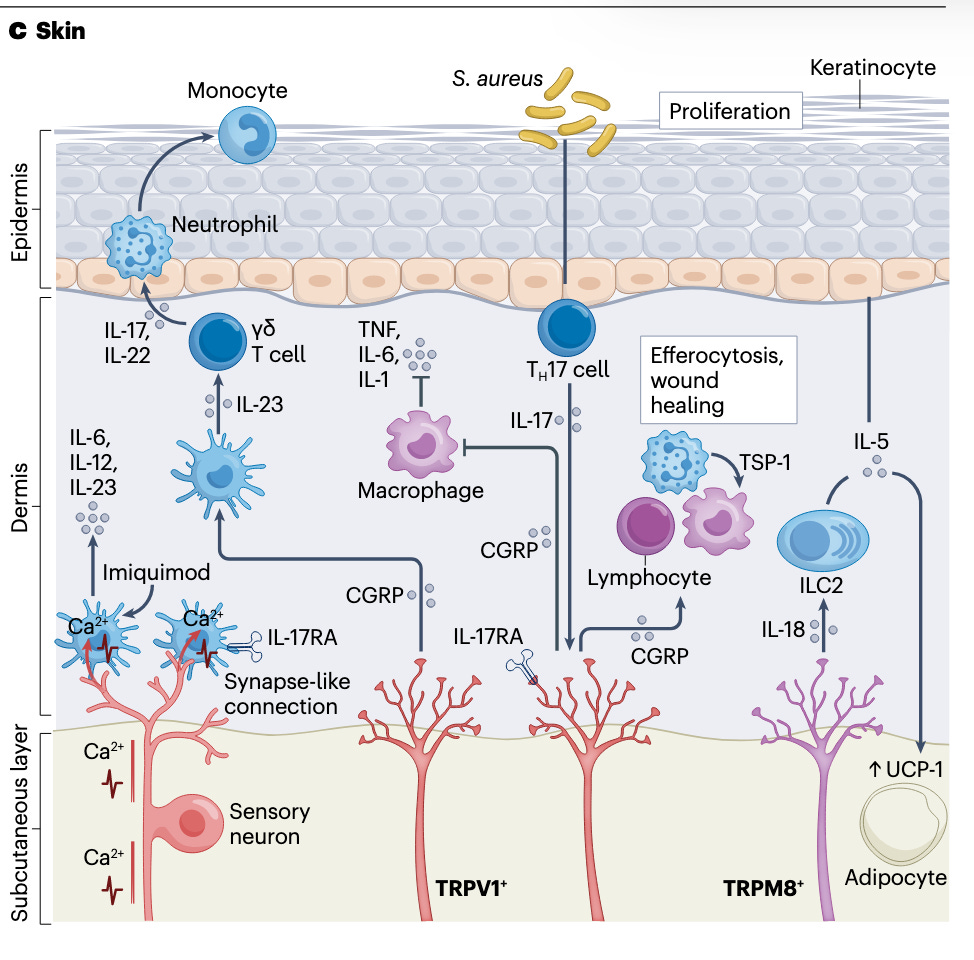
Even the lungs have neural-immune crosstalk: parasympathetic and sympathetic nerve fibers influence immune cell recruitment during asthma or infection. These examples show that the nervous system is woven into the immune response across the body’s organs.
Now we know there are resident immune cells inside the brain, immune populations stationed at its borders, and that the brain can call in extra immune cells from the rest of the body when needed.
In other words, the immune system is not an occasional visitor to the brain; it is a constant presence, integrated into how the brain develops, functions, and responds to change.
Given the range of functions these cells perform, it’s not surprising that when communication between the brain and immune system breaks down, the consequences can be wide-ranging and severe.
What happens when neuroimmune communication goes wrong
In fact, many of our most stubborn health problems—deadly, mysterious, or lacking good treatments—involve this cross-system conversation going wrong.
Neurodegenerative diseases
Alzheimer’s (6-7 million new cases every year), Parkinson’s (more than 10 million people worldwide are estimated to have Parkinson’s), and Huntington’s all feature chronic neuroinflammation. These diseases affect millions annually, yet we still have no cures.
A common thread in many of these neurodegenerative diseases (including Alzheimer’s, Parkinson’s, ALS, and Multiple Sclerosis) is dysfunctional microglia. When these brain-resident immune cells stop working as they should, they can fuel harmful inflammation and accelerate damage to neurons.
We still don’t have the full picture of how these diseases unfold, but we’re starting to connect the dots. Genetics is one piece of the puzzle. For example, ApoE4 (the strongest known genetic risk factor for Alzheimer’s) changes how microglia respond to amyloid plaques and may speed up the spread of toxic tau proteins.
Psychiatric disorders
The immune system also has a powerful influence on mental health.
One clear example comes from cancer patients treated with interferon-alpha, a pro-inflammatory immune signal used as therapy. By ramping up inflammation, the treatment often triggers depression, which is evidence that inflammation itself can alter mood. Indeed, depression is strongly linked to inflammation.
As mentioned earlier, in chronic social stress experiments, immune cells called CCR2⁺ monocytes travel from the blood into the nucleus accumbens (a brain region tied to motivation and reward). There, they release an enzyme called MMP8 that reshapes the tissue scaffolding around neurons, disrupting their connections. The result is changes in brain wiring linked to depression and anxiety-like behaviors.
Together, these findings suggest that stress-induced immune activity mirrors your mood and can reshape the brain itself, influencing how you think, feel, and behave.
Autoimmune disorders
Sometimes the immune system outright mistakes brain tissue for an enemy.
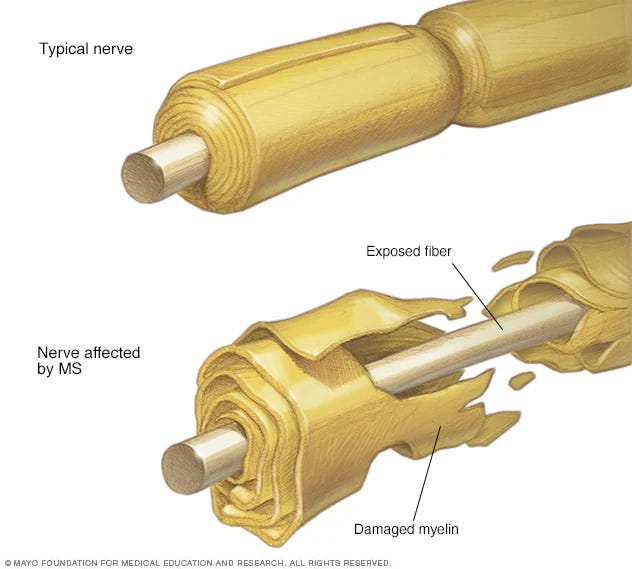
In multiple sclerosis (MS), immune cells strip the myelin insulation off neurons, slowing or blocking the electrical signals they carry. The result can be muscle weakness, vision problems, and difficulty with coordination.
In a disease known as autoimmune encephalitis, the immune system makes antibodies that latch onto neuronal proteins and scramble their signals. One of the best-known cases is journalist Susannah Cahalan. In her 20s, she began experiencing paranoia, hallucinations, and seizures.
At first, doctors thought it was a psychiatric illness. Eventually, tests revealed antibodies against NMDA receptors (a key communication channel in the brain). Once she received immunotherapy, her symptoms reversed. Without recognizing the immune–brain connection, she might never have recovered.
Her story is told in Brain on Fire, available as both a memoir and a film for those curious to learn more. Cases like Cahalan’s show how powerful neuroimmune knowledge can be for solving medical mysteries and for shaping new treatments.
How can we apply neuroimmune insights to medicine?
Scientists are now trying to use this cross-talk to our advantage.
One approach is to stop harmful immune cells from entering the brain. We already do it in multiple sclerosis, where a drug called Natalizumab blocks the adhesion molecules immune cells use to cross the blood–brain barrier. This is neuroimmunology in practice: controlling brain disease by controlling immune traffic. Trials are now asking whether the same trick could ease depression or slow down Alzheimer’s.
That’s one direction: change the immune system and watch what happens in the brain in multiple sclerosis. The other direction—change the brain and watch what happens in the immune system—can be just as powerful. Devices that stimulate the vagus nerve can calm inflammation in rheumatoid arthritis patients who haven’t responded to drugs. Researchers are now exploring whether this nerve-based therapy could work for conditions like inflammatory bowel disease or sepsis.
And sometimes the answer lies in repurposing existing drugs. JAK inhibitors, built for cancer, also block JAK1 in neurons and are being tested for severe itch. Even everyday drugs for high blood pressure, like beta blockers, have a surprising immune effect: they change how stem cells in the bone marrow release new immune cells into the blood.
Conclusion
Real-world problems don’t neatly lie within territorial boundaries.
— Charlie Munger
The same holds for biology. For too long, the brain and the immune system were treated as separate territories, each studied in isolation.
Neuroimmunology shows us that the borders are porous and that breakthroughs often come when we stop respecting those imaginary lines. And maybe, by understanding and deliberately shaping this cross-system conversation, we’ll one day be able to change the course of some of the most challenging diseases we face today.